Elijah Spina
Biography
I graduated in 2009 from the State University of New York, Oneonta with a B.S. in Biological Sciences and a minor in Chemistry. I earned numerous distinctions including Magna Cum Laude and Honors in Biology. While at Oneonta, I conducted research in green chemistry and organic synthesis from 2007-2009 in Dr. Jacqueline Bennett's research group. Our goal was to develop solvents that enabled difficult chemical reactions to occur under low energy conditions with almost no unwanted byproducts. My interests soon shifted toward biomedical research and I obtained a volunteer internship at Columbia University Medical Center in the summer of 2008 studying bone pathology in the lab of Dr. John Manavalan. During my last semester, I also participated in another research project attempting to clone fatty acid transport genes from Caulobacter crescentus in Dr. Fred Zalatans lab. After graduation I was hired as a consultant at New Hope Fertility Center in Manhattan where I identified ways to improve embryo transfer cycle outcomes while continuing to volunteer as a lab assistant. After a few months I was able to devote my time entirely to working in Dr. Manavalan's lab until starting the PhD program at UCSB in 2011.
Research Area
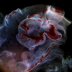
A few striking examples of regeneration hold an almost mythical status among the wonders of biology, such as the arms of sea stars and the autotomic loss of a lizards tail. These dramatic examples are viewed as rare because they signify one end of a continuum represented largely by model organisms with limited regenerative capacities. This and other factors contributed to a lack of mechanistic knowledge about regenerative processes at the cellular/molecular level despite long standing interest. However, progress in evolutionary developmental biology, genomics and stem cell biology have enabled those old questions to be answered. Simply put, the overarching question has been reduced to "Why can some animals regenerate lost body parts while others cannot?". By comparing gene expression between regenerative model organisms such as Ciona and humans, we aim to identify potential targets that can be used to aid the treatment of trauma victims and degenerative diseases. To contribute to this exciting and growing field, I'm using RNA sequencing to investigate transcriptome wide changes in gene expression during regeneration of the oral siphon. In collaboration with Dr. Ken Kosik's laboratory, we've sequenced mRNA and microRNA during multiple stages of regeneration. Finally, to confirm the importance of our results, I'm developing methods to maximize the high throughput and live imaging capabilities of Ciona for assessing the localization and function of genes in vivo.