Network re-wiring in disease
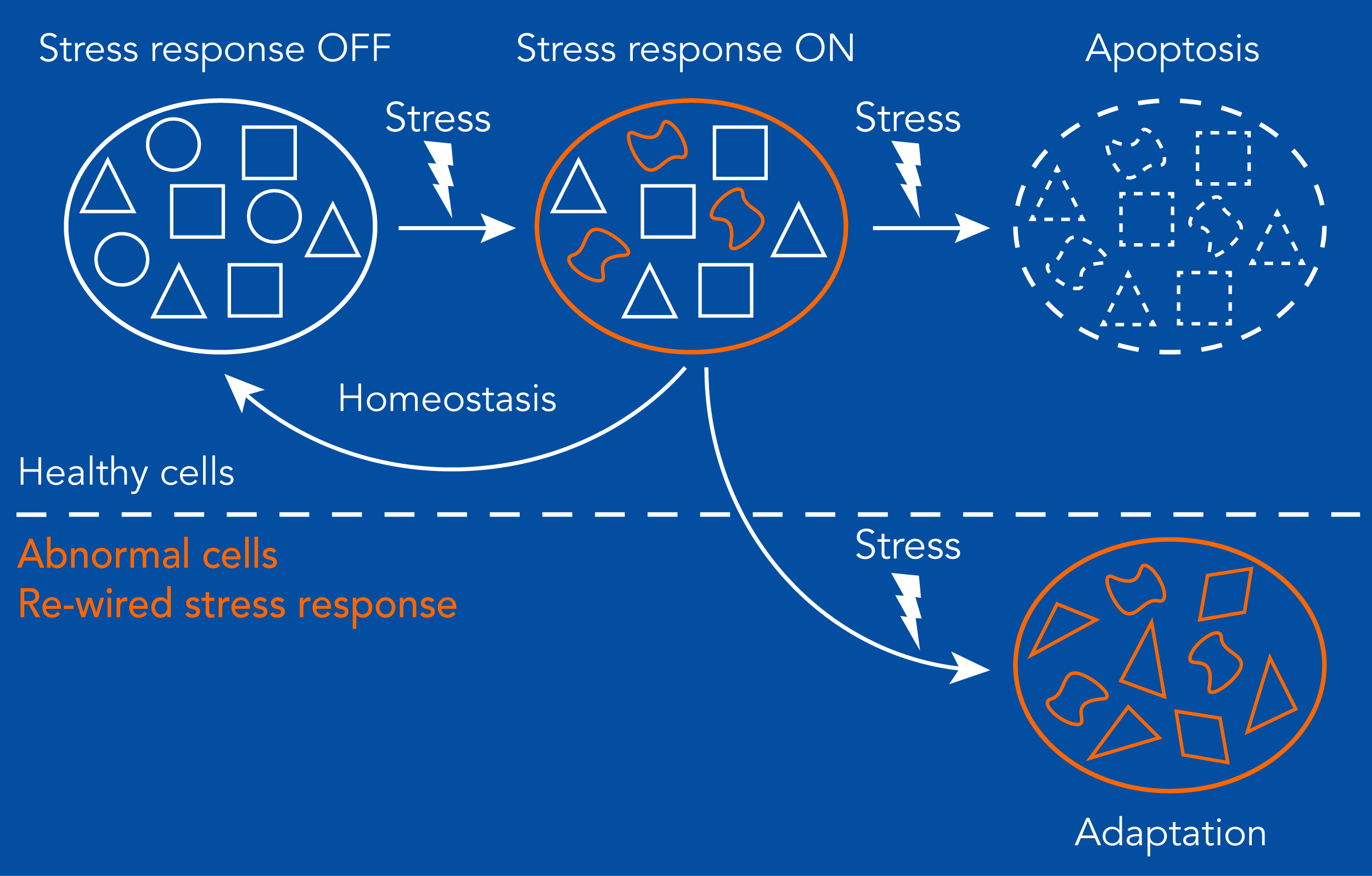
All cellular stress responses safeguard from phenotypic changes that manifest as disease. In disease, these networks may be re-wired to evade or disable apoptotic programs, giving a survival advantage to abnormal cells. A main goal of our lab is to understand in mechanistic detail how these fundamental cellular stress response networks are wired in healthy cells to understand how are they wired in disease.
In disease, cells adapt to chronic stress, generating potential Achilles’ heels that can be exploited for developing new therapies. Multiple myeloma, a rare and devastating cancer of plasma cells, is an outstanding example that powerfully illustrates this concept. By virtue of their genetic makeup, their secretory nature, and their abnormal and uncontrolled growth rate, these cancer cells rely heavily on the proteostasis network –a collection of pathways that oversees the synthesis, folding and degradation of proteins– for survival. Multiple myeloma cells are also exquisitely sensitive to proteasome inhibitors. These drugs are used in the clinic to treat multiple myeloma, and while successful, all patients eventually develop resistance to proteasome inhibitors, suggesting the cancer cells find ways to divert the burden of high protein turnover to alternative mechanisms in order to survive. Using a comprehensive shRNA-based genetic screen, we have recently shown that such network-level rearrangements can promote adaptation when myeloma cells are challenged with proteasome inhibitors used in the clinic (Acosta-Alvear, et al., eLife 2015). By identifying which nodes in the network confer resistance or sensitize to the drug, our genetic screen predicted drug-drug interactions and biomarkers of response to therapy in patients.
We continue to work on the fundamental question of defining the mechanisms by which re-wired cellular stress response networks become rogue cytoprotectors in disease. We pursue the concept of network re-wiring in disease taking the unfolded protein response and the RNA stress response as paradigms. By discovering the dynamic interactions between the several hundred molecules that interface with these stress response networks we can begin to understand their wiring in abnormal cells. This knowledge can pave the way for developing new therapies for several diseases in which cellular stress responses are remodeled, such as cancer, neuropathologies, or viral infection. By systematically dissecting the network and meticulously probing the mechanisms by which these interactions impact cell survival and death, we strive to make fundamental discoveries in cell biology.


