Abstract
Background: Autosomal-dominant polycystic kidney disease (ADPKD) is a common hereditary form of chronic kidney disease with limited pharmacological treatment options. Emerging evidence suggests that metabolic interventions, including ketogenic dietary strategies and reducing lithogenic risk, may positively influence disease progression.
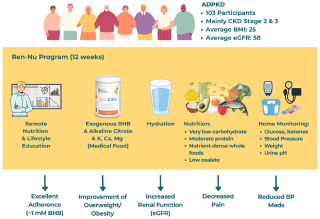
Objectives: This study evaluated real-world outcomes from the Ren-Nu™ program, a remotely administered educational program for individuals with ADPKD that combines the use of a medical food with nutrition and lifestyle changes, including a very-low-carbohydrate ketogenic diet and reduction of renal lithogenic stressors. Ren-Nu™ was launched in 2021 as a collaboration between scientists and nutrition experts from the University of California Santa Barbara and Santa Barbara Nutrients, Inc, and dietitians from Kidney Nutrition Institute.
Methods: Data from 103 ADPKD participants who completed the Ren-Nu™ program between 2021 and 2023 were analyzed in this longitudinal, baseline- controlled evaluation. The 3-month intervention included structured dietary education, regular dietitian and nutritionist support, and supplementation with KetoCitra®, a medical food providing beta-hydroxybutyrate (BHB), citrate, minerals, and alkali base. Primary outcomes included estimated glomerular filtration rate (eGFR), body mass index (BMI), anti-hypertensive medication usage, and self-reported symptom burden. Safety was assessed through routine metabolic biomarkers.
Results: Participants demonstrated high adherence to nutritional ketosis, leading to a significant improvement in BMI. Renal function significantly improved, showing an eGFR increase of 6.3% (from 58.4 to 61.6 ml/min/1.73 m2; P < 0.001). There was a notable decrease in anti-hypertensive medication use and significant reductions in self-reported kidney pain and headaches. Safety markers, including lipid profiles, electrolytes, and acid-base balance, remained stable throughout the intervention.
Conclusion: Use of KetoCitra® supported by nutrition and lifestyle changes in the Ren-Nu™ program demonstrates feasibility, safety, and clinically meaningful improvements in metabolic health, renal function, and quality of life in individuals with ADPKD. These findings support the potential of supplementation, nutrition, and lifestyle interventions targeting metabolic dysregulation and lithogenic risk factors as a strategy in ADPKD management.